Dentsu Integrates BranchLab’s AI-Native Platform, Pathwai™ to Transform Data into Audiences for Healthcare. Read more
Patient, HCP, and Caregiver Exploration
Explore and discover relevant audiences without exposing Protected Health Information.
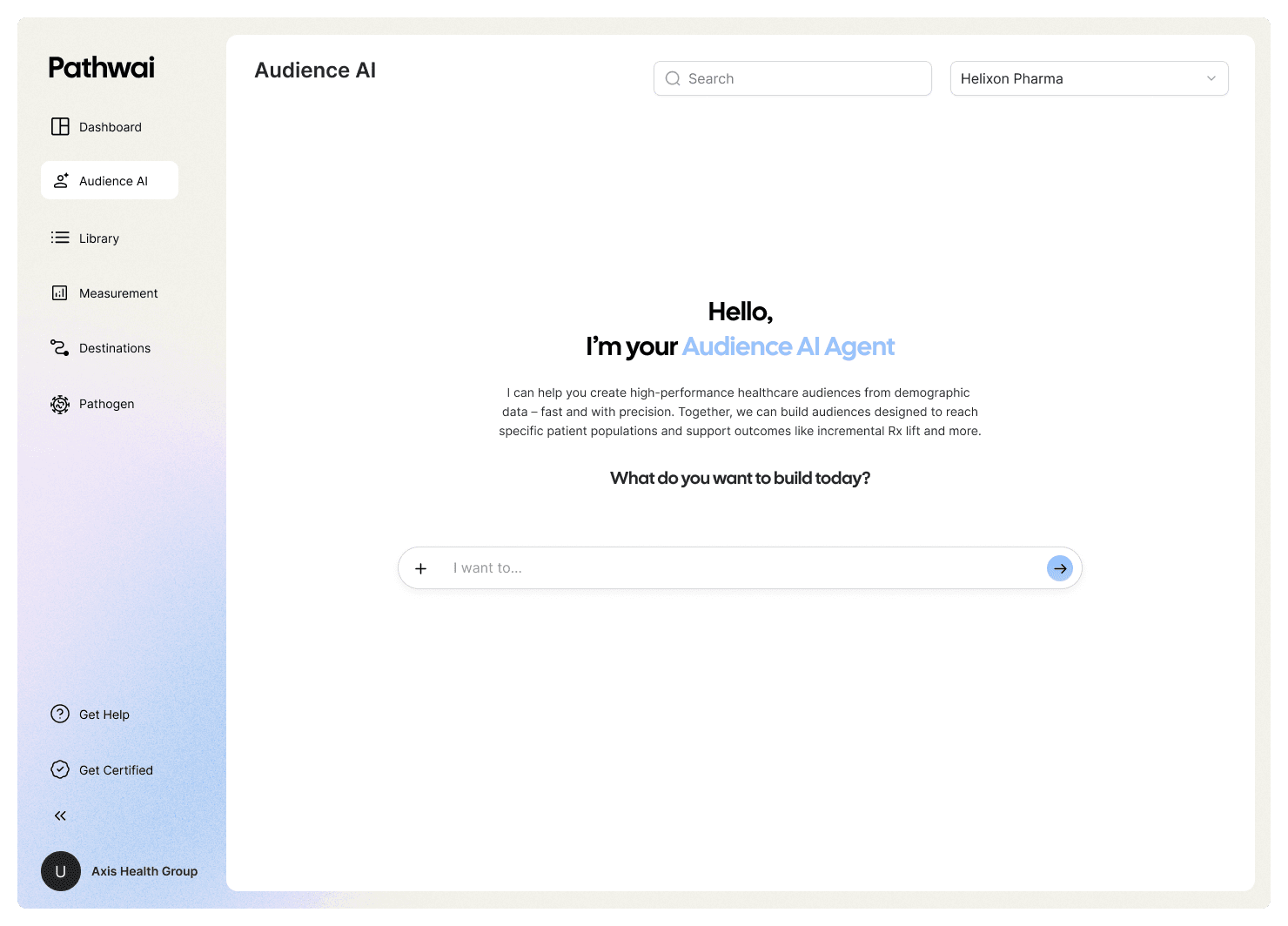
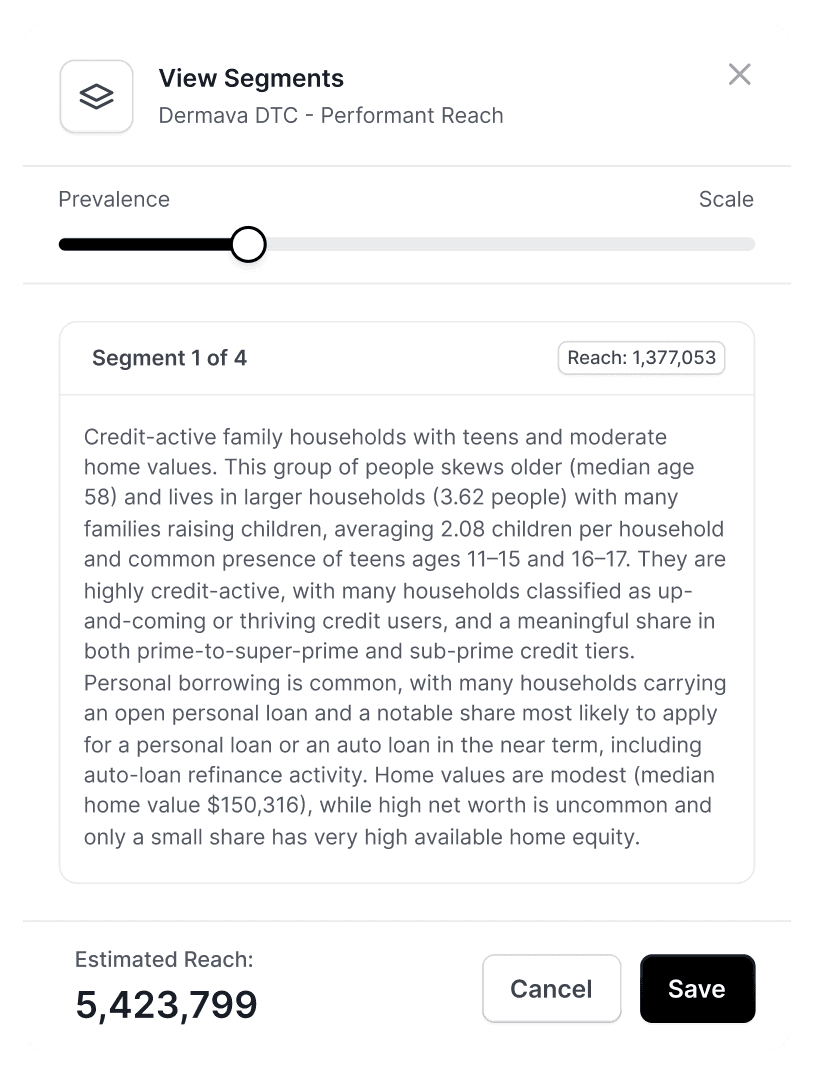
Agentic Audience Creation & Optimization
Design and refine audiences using AI-driven insights.
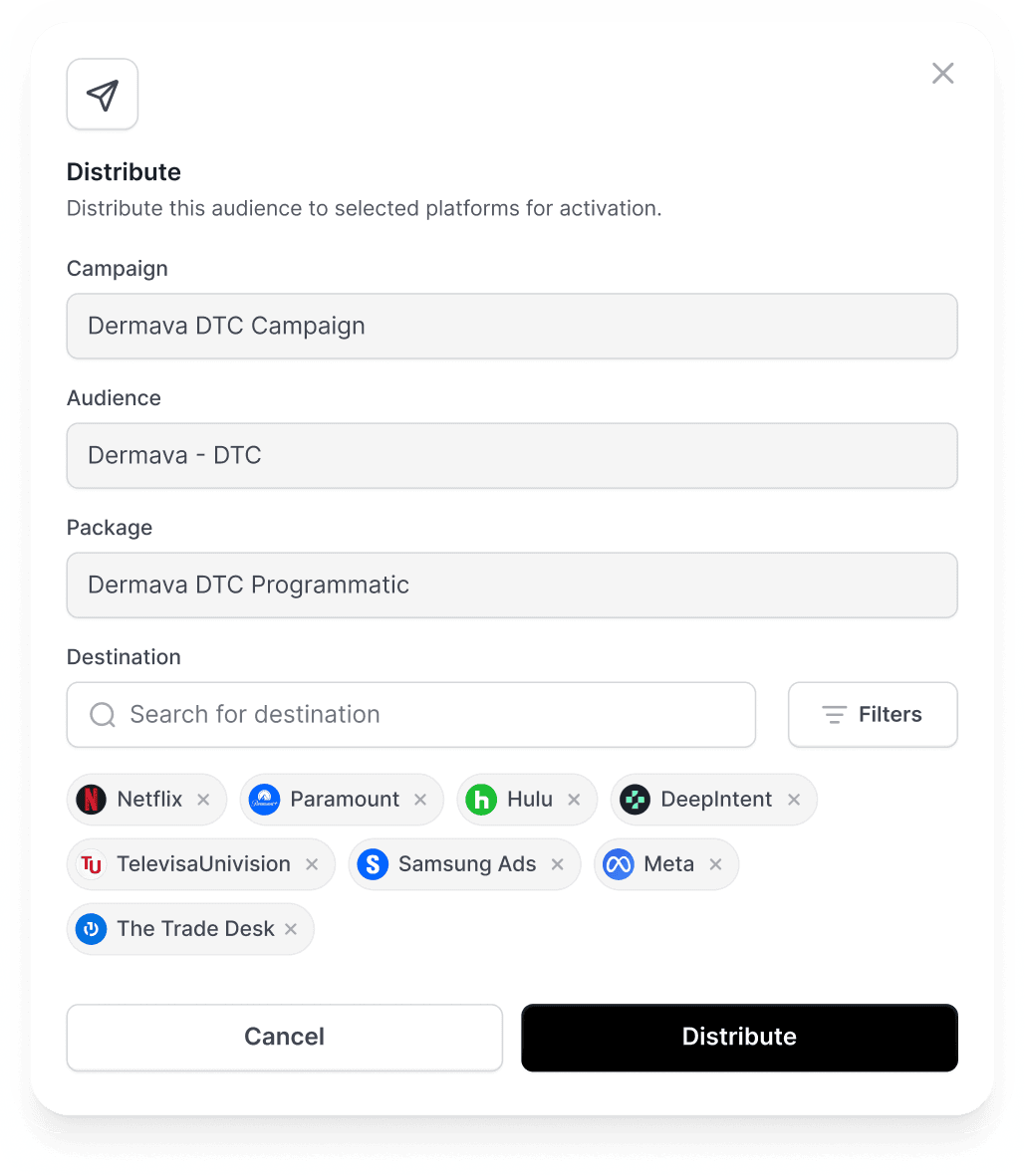
Omnichannel Activation
Deploy audiences across all media types and channels.
Outcomes Optimization & Learning
Measure real-world impact and continuously improve.
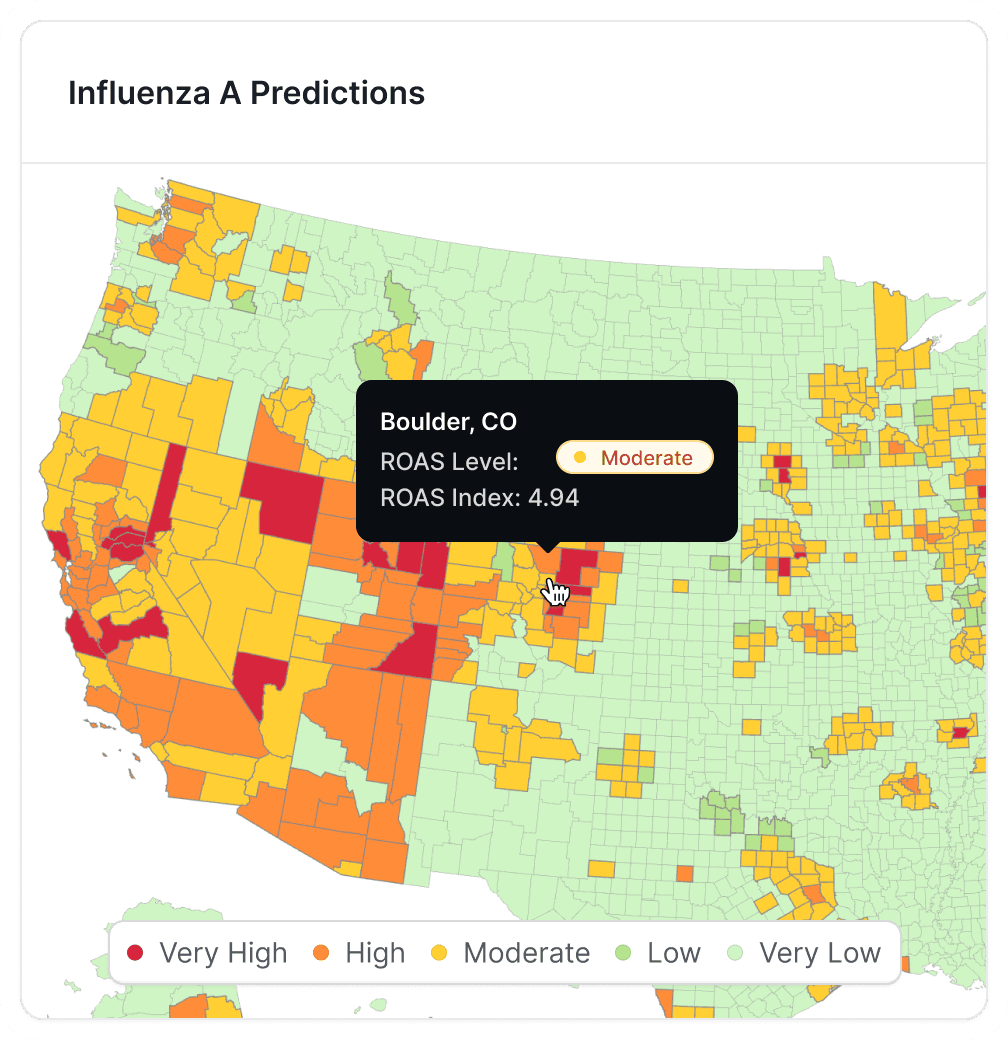
Can AI deployed in demographic data perform like health data?
The answers are in the numbers.
faster
time to execute healthcare campaigns by replacing fragmented workflows with a unified AI-driven decision-making platform.
higher performance
than industry average audience quality when compared to claims-based targeting approaches.
lower CPM
compared to competitive predictive audience targeting using AI-driven predictive models instead of zero-party or claims-based approaches.
reduction
in cost per target audience reached, achieved through predictive modeling instead of traditional targeting models.
“A pivotal advancement in healthcare marketing.”
Brad Fox
SVP, Dentsu Health